
Gunther
Forum Replies Created
-
Gunther
MemberMarch 7, 2019 at 4:15 pm in reply to: How to formulate a properly foaming cationic cleansing conditioner?ngarayeva001 said:Sarcosinate is anionic too… I am sure you will find a use for it, but it probably won’t work with BTMSAin’t Sodium lauroyl sarcosinate supposed to be compatible with cationics like CETAC or Behentrimonium?
https://www.crodapersonalcare.com/en-gb/products-and-applications/product-finder/product/170/Crodasinic_1_LS30 -
No offense, but whenever I read “natural preservatives” or “DIY sunscreen SPF”
I feel like hearing ‘watch this’ in fail videos. -
You can neutralize EDTA (free acid) with Sodium hydroxide to make disodium or Tetrasodum EDTA as desired.
But EDTA should be cheap and widely available.
You many need to ask for its brand names like Trilon B (and generics)
https://www.ulprospector.com/en/na/Cleaners/Detail/3690/83903/TRILON-B-POWDER -
How do you know for sure that glycerin is 15%?
Did you have the product tested at a chemical or chromatography lab?My guess is that glycerin is more like 5%
-
Gunther
MemberMarch 7, 2019 at 3:32 pm in reply to: how much ethanol results in a dangerous good for cosmeticsLots and lots of perfumed body splashes and body mists contain alcohol and they aren’t too iritating or dangerous (always add a flammable sign in the label, just in case)
You can estimate the flash point by looking at some charts like
https://dl.uctm.edu/journal/node/j2010-1/2_Mariana_19-24.pdf
but keep in mind that many fragrance ingredients are flammable too. -
Gunther
MemberMarch 7, 2019 at 3:19 pm in reply to: Effect of PEG40 hydrogenated castor oil on thickening of shower gel formulationI believe the glucoside is the main reason it won’t thicken, and not (or not much) the fragrance and PEG-40 HCG.
Glucosides are notorious for thinning down formulations.Consider replacing coco sulfate with something milder.
Coconut oil is about 50% lauric acid,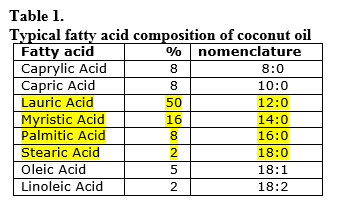
https://www.hebebotanicals.co.nz/sodium-coco-sulfate-another-synthetic-detergent/
so coco sulfate is about 50% SLS = irritating. -
Make sure your dreams don’t become pipedreams
https://chemistscorner.com/cosmeticsciencetalk/discussion/5488/congratulations-on-the-recall-when-marketing-makes-products-less-safe -
JonahRay said:Doesn’t the regular sealing process trap air at the sealed end of the tube to allow the product to be squeezed out?
I don’t think they do.
If they did, the air will be the first thing to come out, with disgusting bubbles or even a farting sound. -
Gunther
MemberMarch 4, 2019 at 2:55 pm in reply to: Need help for my shampoo formulation or new farmulation1. Is that 10% active SLES or 10% of 70% SLES gel?
2. Consider dropping SLS as it’s too drying.
3 Consider reducing SLES to 8% total active, but no less than that as it will be hard to thicken.
4. Drop the olive oil, or reduce it to claims ingredient levels 0.01%
5. Drop the Titanium dioxide, it will just sink to the bottom.
6. Get a ready-made commercial pearlizer (i.e. Euperlan) instead of plain glycol distearate.
7. Drop the cetearyl alcohol
8. What’s glucose de?
9. Drop the propylene glycol
10. Add some CAPB, that will improve foam and help reduce the salt required for thickening it.
11. Reduce the honey content even further to 0.01% claims ingredient levels.
12. Increase Sodium benzoate preservative to 0.5% -
Agree, too harsh for skin use.
albeit sometimes I wonder if SLS is just as harsh, if not harsher than sulfonate.As a nice experiment, you can try neutralizing LABSA with Magnesium hydroxide and see if the resulting Magnesium sulfonate remains water soluble.
That would make it milder, like Magnesium laureth sulfate vs SLES. -
CETAC Cetrimonium chloride works as an emulsifier and it sharply drops viscosity.
But you’d need to get rid of anionics like the SLES containing Lanette SX. -
For low viscosity formulations, something like this may work fine for very small volume production

-
Did you mean tubes like these?

If so, then there are some sturdy, metal “syringes” that work fine and are cheap.
Make sure you do NOT have an airthight seal, as you want air to be able to escape.
Such “syringes” may need an extension nozzle tube to reach the bottom of the cosmetic tube to begin filling it from the bottom, as to avoid trapping air.Some of them even have a caulk gun grip
Something like this, only with a longer outlet tube
-
So I thought, slurries are unavoidable with Polyox
Thanks a lot guys.@Bill_Toge @pepe may I ask what glycerin to Polyox ratio did you use?
I think I’ll try glycerin first, as it doesn’t seem to worsen foam and viscosity as much as propylene glycols or alcohols do. -
Mrkhan said:Gunther is there any side effect of table salt?
So far, only having trouble with its tiny grains of sand becoming noticeable.
-
Gunther
MemberFebruary 28, 2019 at 10:27 pm in reply to: How to suspend dimethicone in SLES based shampoos?chemicalmatt said:Don’t know about “suspension” of dimethicone, but the classic method of incorporating these into shampoo systems used for many decades is to employ a little sodium xylene sulfonate (40% is RM standard) along with a medium-high mw dimethicone, say 1000 - 10,000cst. Even better: add low mw polyquaternium-10 (JR-400) to the shampoo and your deposition will be 500% better. or, stop asking us and just read Des Goddard’s book. (How often do I have to tell people?)Thanks
May I ask what Des Goddard book are you referring to?
He seems to have written several books. -
This study hints that quats/polyquaterniums might increase silicone deposition.
A preliminary investigation of the interaction of a quat with silicones and its conditioning benefits on hair.
SHRENIK NANAVATI and ANNETTE HAMI
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.542.6263&rep=rep1&type=pdfBut there are some limitations to be aware:
1 It used Tricetylmonium chloride (Varisoft TC-90 ), instead of common use Polyquaterniums 7 or 10.
2 It was a semi leave on study as they dipped in test tresses, squeezed them to remove excess liquid, but were NOT rinsed. -
I don’t think Sodium bicarbonate can saponify oils or fats, and if it does, the reaction will be too slow and possibly require higher temps and maybe pressures.
On the other hand, baking soda can well neutralize free fatty acids.
As to why, for ester hydrolysis that is breaking up oils and fats back to free fatty acids and glycerin, you need a strong acid or base and baking Soda ain’t strong enough.
https://en.wikipedia.org/wiki/Saponification#Mechanism_of_base_hydrolysisWhile you could easily hydrolyze oils yourself with hydrochloric acid, purification may become troublesome, so it might be better to buy ready-made distilled fatty acids.
By the way, you can try neutralizing fatty acids with ammonium carbonate, as they feel WAY milder than Sodium or Potassium soaps.
-
Gunther
MemberFebruary 26, 2019 at 7:57 pm in reply to: How to make a cleanser with Decyl Glucoside better at removing makeup?Thanks @ngarayeva001
Are there any such commercial products sold in twin container, twin dispensing nozzle bottles?
As to avoid shaking before use. -
Gunther
MemberFebruary 26, 2019 at 4:29 am in reply to: How to suspend dimethicone in SLES based shampoos?Belassi said:Why would you want to destroy the foaming capability by adding dimethicone?You were totally right, this study shows that dimethicone reduces foam a lot lot.
Silicones as conditioning agents in shampoo
Kazuyiki Yahagi
http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=B1BB72C01A6C21C29D10EC5D08C4910A?doi=10.1.1.516.2089&rep=rep1&type=pdf
This may explain why so many big brand shampoos add SLS to SLES, to boost foam lost by dimethicone. -
I don’t know for sure, but I believe that it shouldn’t be Iodized in order to meet USP grade.
http://www.usp.org/sites/default/files/usp/document/harmonization/excipients/sodium_chloride_monograph.pdf -
Gunther
MemberFebruary 25, 2019 at 8:16 pm in reply to: Emulsifying Cleansing Oil with Polyglyceryl-4 OleateCheck out this thread where @ngarayeva001 cleverly mentioned that makeup is quite waterproof, thus water based formulations won’t (usually) remove makeup very well.
https://chemistscorner.com/cosmeticsciencetalk/discussion/5553/how-to-make-a-cleanser-with-decyl-glucoside-better-at-removing-makeup -
Gunther
MemberFebruary 25, 2019 at 7:37 pm in reply to: What prevents water soluble silicones from ending up in the drain instead of on hair?Perry said:I think most of it does just get rinsed down the drain. This was always a problem I had with using water soluble silicones in rinse off hair products.In my experience, I was never able to demonstrate a significant effect via tress testing on a blinded basis. We mostly included the material because it affected the feel of the product while in use. I don’t recall seeing any post-use benefits.
I agree. Pretty much anything ends up in the drain in a rinse off product.
Do you think quaternized silicones, like amodimethicone get a better chance since they’re kind of “attracted” to hair? -
May I ask what’s the purpose of adding pentylene glycol?
BTMS-50 contains butylene glycol, so maybe you don’t need any pentylene glycol? -
In my opinion, silicones leave a noticeable, smoother feel than polyquaterniums do.
But most silicones need expensive solubilizers, or complex suspension procedures.
https://chemistscorner.com/cosmeticsciencetalk/discussion/5545/how-to-suspend-dimethicone-in-sles-based-shampoos
While polyquaterniums just need to be poured in and mixed.

