
em88
Forum Replies Created
-
Pharma said:Here in CH, there are others which contain also 2% and all these are prescription drugs whilst the 1% Terzolin is an OTC shampoo (click HERE).Ketoconazole is, compared to other topical azole-antimycotics the least lipophilic one i.e. easiest to work into a shampoo whilst all others are used in creams or nail varnish.
Nizoral 60 ml shampoo costs like 14 Eur? 😮
-
Maybe NaCl is not the right ingredient to increase viscosity. Not all sufactants react the same when salt is added.
-
Thank you for the clarification,
So if a substance does not have a SCCS opinion and is included in 1B, it can’t be used in cosmetics? -
So anyone has any experience with EC Regulation 1223/2009 on cosmetics?
-
em88
PharmacistMay 17, 2019 at 11:08 am in reply to: A view that Chemists shouldn’t formulate natural skincare. Only cellular biologists.Don’t waste your time with these kind of advertising articles.
-
em88
PharmacistMay 5, 2019 at 11:52 am in reply to: Salicylic Acid and Lactic Acid as preservatives!I don’t think you will need a preservative for both of your formulations. Try to test your formulations in a microbiology lab.
-
Quimico said:Tauriel Use EDTA 0.5% for preservative and salt for thickner.
Xanthan gum is not easily dissolvedEDTA as preservative?
-
To me, it looks like you added in your formula everything you had in front of you.
Have you actually tried this formulation?
Why did you add xanthan gum when you have already 2% of gel emulsifier?
Not sure if you really need Cetearyl alcohol/ceteareth-20, the gel emulsifier should be enough. -
em88
PharmacistApril 5, 2019 at 2:16 pm in reply to: viscosity of dish wash shampoo with Nacl(salt)do the salt curve. There is an article written by Perry, in his blog, regarding the salt curve.
-
Bill_Toge said:that’s a new one on me - amides like urea are usually stable unless you have a lot of alkali present
There are many articles that mention a degradation of urea in NH3 and CO2.
I’ve noticed myself an increasement of pH over time.
In creams is even easier to spot the bubbles of CO2.
https://www.ncbi.nlm.nih.gov/pubmed/25043489
Indeed, taking in consideration that there are covalent bonds, urea should be stable, but in my experience urea was not stable.Bill_Toge said:only urea/formaldehyde condensates, e.g. imidazolyl urea, do thisMy mistake, was thinking about something else.

-
You need a proper emulsifer in you formula, cetearyl alcohol is not an emulsifier.
Regarding low HLB emulsifiers, other alternatives are sorbitan oleate, sorbitan stearate etc
If you really want to have cetearyl alcohol in your formulation, use Cetearyl Alcohol (and) Sodium Cetearyl Sulfate (Lanette N from BASF is an example). -
1) Is it hard to “stabilize”?
Yes
- Does it degrade or precipitate? Under what conditions?
It degrades. Water and temperature presence are enough.
- Does it always change the pH of a product? Are there ingredients to add to prevent pH shifts?
Yes. You need to add a buffer system2) Is there any difference at all between the various forms of Urea: Hydroxyethl, Imidazolidinyl, Diazolidinyl, and Urea USP?
Not every compound with “urea” in the name is urea! Check the molecule to understand.
- Is one form more stable/easier to work with than others?
Urea is only one.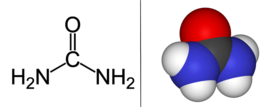
- Do ALL forms of Urea release formaldehyde?
Probably -
Belassi said:Did they consider Cetearyl Alcohol as an emulsifier?
— It IS.So you changed your mind? https://chemistscorner.com/cosmeticsciencetalk/discussion/745/cetearyl-alcohol-emulsifier-or-not
ngarayeva001 said:I am sure it has something to do with the calculation. I have a formula (it has been stable at the room temperature for the last 7 months) emulsified with 3% Glyceryl Oleate and 1% of Polysorbate 60 and it is O/W. Oil pase is 10%. Recalculate the HLB.You might be right.
sorbitan stearate - 2 %, polysorbate 60 - 1.5 % can emulsify an oil phase with the HLB of 9.08
cetyl palmitate rHLB - 10 (3%)
cetearyl alcohol rHLB - 15.5 (10%)
Octyldodecanol rHLB - around 11 (13.5%) accourding to this paper: https://www.researchgate.net/publication/289449490_Required_Hydrophilic-Lipophilic_Balance_Values_of_Octyldodecanol_from_Emulsion_Stability_Tests_and_Relative_Dielectric_Permittivity_MeasurementsDtdang said:Try using emulsifier Sepiplus 400
it is freedom formulation to 50% oulI’m not allowed to change formula, I’m stuck with the same ingredients as the reference.
-
I wrote a few formulas, and I was thinking to try first 8% octyldodecanol.
Just found a clotrimazole cream drug with 13.5% octyldodecanolsorbitan stearate - 2 g, cetyl palmitate, cetostearyl alcohol (cetyl alcohol 60% and stearyl alcohol 40%), polysorbate 60 - 1.5 g, octyldodecanol - 13.5 g, benzyl alcohol, purified waterIt is strange that they added more sorbitan stearate than polysorbate 60 for a o/w cream. Did they consider Cetearyl Alcohol as an emulsifier?
-
ngarayeva001 said:Think of it as of vegetable oil. It’s a medium viscosity ester. Very multifunctional. You can use more than 6%.
That would be great, I can regulate the viscosity much easier.
-
em88
PharmacistMarch 26, 2019 at 6:42 pm in reply to: Online store based in the US sells dangerous cosmeticsAVisotsky said:The domain name though… ISIS ))
)) And gold gTLD. Strange combination
-
em88
PharmacistMarch 22, 2019 at 7:51 am in reply to: Online store based in the US sells dangerous cosmeticsngarayeva001 said:I found even worse:
https://www.isis.gold/product/iris-whitener-change-eye-color/Arbutin eye drops. This is just a crime.
They charge $200 for a bottle?
-
-
sven said:Hi all. Crodex A seems to be made up of cetostearyl alcohol and SLS. Anybody know what % the various components are? I am not able to find this on the croda literature i have. Client really insist on using this as an emulsifyer for a conditioner.
Thank why not use it?
You should ask the CoA for Crodex A. Most likely they list some wide rages for each component of that blend
. -
em88
PharmacistMarch 7, 2019 at 3:50 pm in reply to: how much ethanol results in a dangerous good for cosmeticsIn europe there are no restrictions http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=74174
-
em88
PharmacistMarch 7, 2019 at 9:39 am in reply to: how much ethanol results in a dangerous good for cosmeticsIt depends on where you cosmetic gel is applied. Normally 15% ethanol is fine.
-
Even if you have the HLB, how are you going to calculate the amount of surfactant formed?
-
If the acid from the potassium salt is weaker than the fatty acid than it should form a soap. It is the same principle as with borax, which is a sodium salt with a weak acid.

