
Gunther
Forum Replies Created
-
Cetyl alcohol is insoluble in water
so unless it’s part of an emulsifier system, it will slowly sink to the bottom. -
Gunther
MemberJuly 17, 2018 at 3:31 pm in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?It held fine in 5ml
but wow I’ll see if I can make a more concentrated solution.For 100 ml
0.4% methylparaben Sodium salt = 400 mg
0.19% propylparaben Sodium salt = 190 mgAccording to this
http://www.nardev.com/UploadSection/ProdCat-184-1444197298.pdf
their solubilities in water @ 25 C are:
methylparaben Sodium salt 80g /100 ml
propylparaben Sodium salt 100g / 100 mlSo
400 mg methylparaben Sodium salt needs 0.5 ml water to dissolve
190 mg propylparaben Sodium salt needs 0.19 ml water to dissolve
total 0.69 ml
(and the afore mentioned 147.34 mg NaOH)So it’s possible to make a EU-regulations complying paraben Sodium salt concentrate where you just need to pour 1 ml of parabens concentrate, to preserve 100 ml of formulation.
-
Gunther
MemberJuly 17, 2018 at 3:18 pm in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?Thanks guys I feel flattered for the synthesis comments,
even though it’s technically synthesis, it’s a really simple one.@Microformulation thanks
even tough Sodium salts of parabens are available worldwide, just not here (Central America, Latin America), so I tried to see if I can avoid int’l shipping costs.
Same for dishwashing sulfonates, they are available, but linear sulfonic acid + NaOH is cheaper.@Sibech
1) I reacted them together as I wanted a ready-made paraben mix with the proportions allowed in EU for methyl and propylparaben.
They can be reacted separately
You can allow water to evaporate for dry Salts.
2) You’re right. They may hydrolyze over time, so it makes sense to make small batches, to be used quickly.
3) I surely will. -
There’s an article that says that too much SLES has relatively low foam with small bubbles
too much SLS causes stringy flow
SLS:SLES 3:7 ratio seems to work better
https://www.happi.com/contents/view_features/2009-09-02/the-formulation-basics-for-personal-cleansers -
pH 10-11 will damage skin or even hair on contact
it’s irresponsible to sell such a product, and you’ll likely get sued or prosecuted.Just too dangerous for consumers, even for a concentrate product, supposed to be diluted before use.
-
Gunther
MemberJuly 16, 2018 at 3:50 pm in reply to: Formulating Baby Wash (losing viscosity after 24hrs)Drop the glycerine
Allow 72 hours to get a final viscosity.
-
Gunther
MemberJuly 14, 2018 at 6:06 pm in reply to: Can silicones be emulsified in ethyl alcohol for a hairspray?By adding BTMS+silicones it’s Refractive index has sharply increased over that of alcohol+PVP alone
makes a nice magnifying glass effect in the clear PET pump spray bottle.
-
Gunther
MemberJuly 14, 2018 at 5:57 pm in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?Most of it dissolved within 15 minutes! = success

I poured
5 ml demineralized water
150 mg Sodium hydroxide flake (solid)
400 mg methylparaben
190 mg propylparabenI didn’t wait for the Sodium hydroxide flake to dissolve
I just poured everything the above ingredients in that order
as expected, parabens didn’t dissolve at first
but within 15 minutes (I wasn’t checking it, I went to prepare myself a cup of coffee), most dissolved with little stirring, no heating neededOnly a few, tiny granules remain undissolved on the bottom of the test tube.
I wonder if they’re impurities. Will leave them over the weekend with occasional stirring to see if they dissolve.
If not, will just filter them out.I will repeat the test by the next week, and check its pH.
But so far, a working aliquot of predissolved paraben preservatives seems perfectly workable,
… and it’s truly self preserving lol -
Gunther
MemberJuly 14, 2018 at 5:45 pm in reply to: Can silicones be emulsified in ethyl alcohol for a hairspray?So far, everything seems to have properly dissolved overnight
I still filtered it, just in case.The next time, I will try to make to make the silicones-in-water phase with twice as much silicones to see if it holds
7% BTMS-50
14% silicone blend.https://chemistscorner.com/cosmeticsciencetalk/discussion/4492/btms-50-to-silicones-ratio
-
To make it worse, it must pass challenge tests
remember this ain’t a pharmaceutical or food product that is readily consumed after opening
rather it can be left opened for months before all it’s used up by the customer.
So proper preservatives are unavoidable.P.S. I believe there’s either a limit on how much salicylic acid you can use skin applications, there must be warning on the label.
-
Gunther
MemberJuly 14, 2018 at 5:34 pm in reply to: What is my baking soda and citric acid reacting to?Sorry, but Sodium carbonate Na2CO3 still makes water upon reacting with citric acid (or any other carboxylic acid, or even inorganic acid)

Hypotethically, an anhydride could be used instead of citric acid
https://en.wikipedia.org/wiki/Organic_acid_anhydride
but they’d be too expensive
http://www.alfa-chemistry.com/cas_24555-16-6.htm
and who knows if they’re safe for OTC skin applications, even if readily hydrolyzed by water to their parent acids.If achieving a long shelf life is really desired (even Lush bath bombs fail to do so) the best approach would be separate compartments for citric acid and bicarbonate, with a divider that either dissolves itself in water, or its pushed to rip by the customer, just before use.
After all grenade-shaped bath bombs have been tried before
https://youtu.be/GKJVtMcaEKk
so a lever-activated “fuse” to rip the divider is theoretically workable (remember all parts must water soluble).
If properly done it will sell well in TX, but not in San Francisco CA. -
This Cosmetic and Toiletry Formulations 4 formula uses almost 3x silicone as BTMS.
But it’s just BTMS, not BTMS-50 with butylene glycol.
-
Gunther
MemberJuly 13, 2018 at 10:37 pm in reply to: What is my baking soda and citric acid reacting to?Bath bombs are usually done dry pressed
so you’ll want to warm both citric acid and Sodium bicarbonate separately in a mildly hot plate so moisture evaporates.Most commercial citric is sold as monohydrate form
https://www.sigmaaldrich.com/catalog/substance/citricacidmonohydrate21014594929111?lang=en®ion=USIt can be converted to anhydrous (no water) form by heating it
https://www.sigmaaldrich.com/catalog/substance/citricacidanhydrous192127792911?lang=en®ion=UShttps://www.researchgate.net/post/how_to_convert_monohydrate_citric_acid_into_anhydrous_citric
Dry, pressed citric acid + baking soda won’t last forever
even if properly sealed, with no outside air or water, they can slowly react as its chemical reaction makes water on its own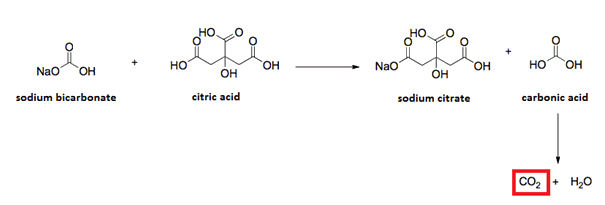
So you can either use/sell the product fresh
Or theoretically separate, yet water soluble “enclosures”, keeping baking soda apart from citric acid.
Some hard soap/synthetic detergent or starch dividers/enclosures can be used. Trial and error is needed to make it work. Please keep us posted with the results. -
Gunther
MemberJuly 13, 2018 at 4:54 pm in reply to: What is my baking soda and citric acid reacting to?While I haven’t made bath bombs myself,
I looks like they’re cross-reacting
so it makes sense to “encapsulate” at least one, if not both citric acid and baking soda inside a water soluble, yet solid ingredient.Also they need to be wrapped inside a water proof film or container.
-
Gunther
MemberJuly 13, 2018 at 4:49 pm in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?Unfortunately Sodium salts ain’t available here, so I would need to import them by plane.
So maybe I can avoid that.As to follow EU regulations
0.4% methylparaben
0.19% propylparaben
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R1004&from=ENI’ll try to react:
400 mg methylparaben
190 mg propylparaben400 mg methylparaben = 400 mg / 152.15 = 2.63 mmol = by 40.00 = requires 105.16 mg NaOH
190 mg propylparaben = 190 mg / 180.20 = 1.05 mmol = by 40.00 = requires 42.18 mg NaOH
Total 147.34 mg NaOHAs for water, 10 ml would be fine, I believe
All Sodium salts will get dissolved
but not much unreacted parabens.0.14734 g in 10 ml
equals 14.734g in 1 L
14.734g / 40.00 = 0.37 M NaOH
So the solution is safe to handle (with gloves). -
Gunther
MemberJuly 13, 2018 at 4:06 am in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?Methylparaben has a 152.15 molecular weight
https://www.sigmaaldrich.com/catalog/product/usp/1432005?lang=en®ion=USPropylparaben 180.20 mol wt
https://www.sigmaaldrich.com/catalog/product/usp/1577008?lang=en®ion=USAnd Sodium hydroxide 40.00 m.w.
https://www.sigmaaldrich.com/catalog/product/mm/30620m?lang=en®ion=USSo, if a stoichiometric ratio concentration is desired, use the above mentioned proportions.
I hope to get test results posted by the next week. -
Gunther
MemberJuly 13, 2018 at 3:50 am in reply to: Pros and Cons of Preserving Lotion with Sodium Benzoate and Sodium Dehydroacetate?In this benzoate vs methylparaben head-to-head study
You can see in Table 1 that Sodium benzoate requires high concentrations, sometimes well beyond those allowed in cosmetics or foodstuff, to properly preserve the product.
i.e. in S. aureus (Clinical) it needs 10 exp 1 =10 mg/ml = 1%
Compare this to Table 2 where the highest methylparaben concentration needed was 10 exp -3 = 0.001 mg/ml = 0.0001%
On the bright side, a Sodium benzoate Pro is that it easily dissolves in water, unlike parabens.
I hope to conduct Parabens + Sodium hydroxide tests results by the next week
https://chemistscorner.com/cosmeticsciencetalk/discussion/4485/has-anyone-reacted-parabens-with-sodium-hydroxide-to-make-paraben-sodium-salts -
1. Is that 10% active SLES or 10% as-supplied?
Is it’s 10% active it’s ok, although 8% is a bit better IMO
if it’s 8% supplied at 70% that’s 7% active, a little low and may cause trouble thickening with salt.2. 3.5% salt is too much, That may well be the cause it’s very thin.
Begin adding salt in 0.25% increments until about the desired viscosity is achieved (it gets at bit thicker in 48-72 hours).3. What’s the foam booster composition?
4. I assume pearls conc is a pearlizer?
5% might be too much. Usually 3% works fine for most pearlizers.5. What’s the preservative composition?
6. 0.2% EDTA is a bit excessive. 0.1% is enough.
-
Cetearyl alcohol and Polysorbate 60 ain’t in Prop 65 list
https://oehha.ca.gov/media/downloads/proposition-65//p65list052518.pdf -
Gunther
MemberJuly 8, 2018 at 4:29 pm in reply to: Has anyone reacted parabens with Sodium hydroxide to make paraben Sodium salts?To gauge if it’s properly reacting
maybe we can use a limited amount of water
so mostly paraben Sodium salts and not much bare parabens will get dissolvedCompare paraben Sodium salts solubility:
Solubility of Paraben Salts within various solvents.
All results in GR/100 ML at 25ºC
Solvent
Water: Sodium Methylparaben 80
Water: Sodium Propylparaben 100
http://www.nardev.com/UploadSection/ProdCat-184-1444197298.pdfParabens
Solubility (gR/100gR) 25º C
Water: Methylparaben 0.25
Water: Propylparaben 0.04
http://www.nardev.com/UploadSection/ProdCat-183-1444197164.pdf -
Gunther
MemberJuly 7, 2018 at 4:03 pm in reply to: Why D5 Cyclopentasiloxane doesn’t show in EU banned/restricted chemicals list?Any chance they may not get banned in leave-on products?
Being leave-on they won’t end up flushed down the drain, they’ll first evaporateUnless they can prove that it condenses back in rainfall and ends up in rivers and oceans before being biodegraded.
I wonder where does it go?
Does it float high up in the atmosphere?
Does it sink just above the ground?
Where does it go?, reminds me of Jack Black and Ben Stiller ‘Envy’ movie.
https://en.wikipedia.org/wiki/Envy_(2004_film) -
Gunther
MemberJuly 7, 2018 at 2:07 am in reply to: Do high molecular weight dimethicones work better in shampoos?This study says that 10,000 to 280,000 polydimethylsiloxanes (cyclomethicones) work better.
I wonder how that molecular weight “translate” to dimethicone viscosity.
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.542.6263&rep=rep1&type=pdf
The study also says that:
- Silicone deposition is dependant on concentration.
- Cetrimonium aids deposition -
A. Proven but regulated:
I believe low concentration minoxidil or ketoconazole shapoo can be sold as OTC, no prescription required, but FDA registration is still needed.B. Unproven and unregulated
Peppermint essential oil
Copper peptides as previously mentioned.
You can Google studies that suggest they might work. Depending on your (lack of) claims, you could avoid FDA registration if sold as cosmetic.

