Home › Cosmetic Science Talk › Formulating › What is my baking soda and citric acid reacting to?
-
What is my baking soda and citric acid reacting to?
Posted by Sophie9 on July 13, 2018 at 3:55 pmDear All,
This is Sofie and I’m a stay at home mom, I’ve made some homemade lotion for a while. But recently i’ve been trying to make bathbombs, they always turned out looking great but after a month or 2, they seemed to expand and crack.
I live in Texas, so I assume it’s the humidity because I don’t use water in it, just 2:1:1 baking soda, critic acid, salt, alcohol and essential oil.
So I stopped making them and decided to try making bathbomb in jars instead, because I like more oil, so I do 20% baking soda, 10% citric acid, 30% sweet almond oil, 39% Sea salt, a few drops of essential oil. And then I place it in jars and close right. But after a few days it seems that the bath paste expands and the oil comes out of the jars. I don’t know what’s wrong, could it be the humidity or the carrier oil is not pure and has water in it? I also notice the older batches I made with less oil also don’t fizz anymore when I use it, could it just be that the baking Soda and Citric acid just react themselves and expire without being contact with water?
Sorry if my questions are stupid, i’m Just experimenting different things.I appreciate your knowledgeable inputs.
Sofie
Sophie9 replied 5 years, 9 months ago 6 Members · 14 Replies -
14 Replies
-
While I haven’t made bath bombs myself,
I looks like they’re cross-reacting
so it makes sense to “encapsulate” at least one, if not both citric acid and baking soda inside a water soluble, yet solid ingredient.Also they need to be wrapped inside a water proof film or container.
-
Hi Gunther, Thank you for your response. Encapsulate one of the ingredients sounds very interesting and new to me. I googled it a bit but couldn’t find how I would do it.
-
Well … Sodium Bicarbonate is a base and Citric Acid is an acid. So, you’re getting a reaction between the Sodium Bicarb and Citric Acid producing water and carbon dioxide.
You can try coating the Sodium Bicarbonate with Oil and the Citric Acid with oil separately before you combine the two to see if that has any effect. Or, just leave the Citric Acid out altogether.
-
Yeah I usually mix all the dry ingredients first. I will try next batch with oil coating as your suggestion and see. Thank you
-
Bath bombs are usually done dry pressed
so you’ll want to warm both citric acid and Sodium bicarbonate separately in a mildly hot plate so moisture evaporates.Most commercial citric is sold as monohydrate form
https://www.sigmaaldrich.com/catalog/substance/citricacidmonohydrate21014594929111?lang=en®ion=USIt can be converted to anhydrous (no water) form by heating it
https://www.sigmaaldrich.com/catalog/substance/citricacidanhydrous192127792911?lang=en®ion=UShttps://www.researchgate.net/post/how_to_convert_monohydrate_citric_acid_into_anhydrous_citric
Dry, pressed citric acid + baking soda won’t last forever
even if properly sealed, with no outside air or water, they can slowly react as its chemical reaction makes water on its own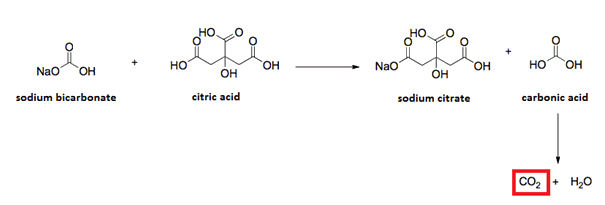
So you can either use/sell the product fresh
Or theoretically separate, yet water soluble “enclosures”, keeping baking soda apart from citric acid.
Some hard soap/synthetic detergent or starch dividers/enclosures can be used. Trial and error is needed to make it work. Please keep us posted with the results. -
I appreciate the informative response. So they react with each other regardless, I was afraid that that was the reason, but i’m glad I got the answer for that because I couldn’t understand why. I might eventually just have to remove one of them from my formula then, because it would be complicated to sell it separately. But I will experiment all the options given first and see.
-
2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
In other words, the sodium bicarb contains water. You could try using sodium carbonate. -
Sorry, but Sodium carbonate Na2CO3 still makes water upon reacting with citric acid (or any other carboxylic acid, or even inorganic acid)

Hypotethically, an anhydride could be used instead of citric acid
https://en.wikipedia.org/wiki/Organic_acid_anhydride
but they’d be too expensive
http://www.alfa-chemistry.com/cas_24555-16-6.htm
and who knows if they’re safe for OTC skin applications, even if readily hydrolyzed by water to their parent acids.If achieving a long shelf life is really desired (even Lush bath bombs fail to do so) the best approach would be separate compartments for citric acid and bicarbonate, with a divider that either dissolves itself in water, or its pushed to rip by the customer, just before use.
After all grenade-shaped bath bombs have been tried before
https://youtu.be/GKJVtMcaEKk
so a lever-activated “fuse” to rip the divider is theoretically workable (remember all parts must water soluble).
If properly done it will sell well in TX, but not in San Francisco CA. -
But you could heat the sodium carbonate to remove the water of crystallisation, yes?
-
“Bath Bombs” are a product that is meant to create a reaction between the acid and alkaline substances of baking soda and citric acid so they dissolve and “fizz” when used in the tub. There must be a certain ratio between the two and the late Maurice Hevey commented on this topic at the “Soap Dish Forum” in 2003 by saying:
“Apr 25 2003, 06:56 AMPost #3
forum guru 





Posts:
3,572
Joined: 24-November 02 When I was a kid, we made bottle bombs. When
When I was a kid, we made bottle bombs. When
you’re young, you are bullet proof. Ah! to be bullet proof again.The
following information was originally part of an article, “Multi-Sensory
Technologies for Today’s Effervescent Bath and Shower Products” wriiten by
Allen Rau. This article appeared in the October 2001 issue of Cosmetics &
Toiletries magazine and presents the basic technology and ingredients used in
the creation of effervescent bath and shower products.“The chemical
reaction that creates the fizz in effervescent bath and shower products is quite
simple. An acid is used to neutralize a carbonate salt. This releases carbon
dioxide (CO2) gas, the salt of the acid, and water. Obviously, the carbon
dioxide gas is the fizzing that characterizes effervescent products.There are a few points here that are not so obvious, but are very
important. First, water is needed to start the reaction. Without water, neither
the acid nor the carbonate can dissociate. If there is no dissociation, the
effervescent reaction cannot start. Once the reaction starts, however, it
generates more water. This means that effervescent products must be carefully
formulated, produced in appropriate environments and packed properly. Otherwise
their inherent instability can ruin them.Specifically, all raw
materials used in an effervescent product must be anhydrous (without water) and
must be stored so that they remain dry. The manufacturing environments where
these products are made must also be designed to maintain dryness. Typically
these facilities are dehumidified to less than 10%RH. To protect them from
ambient humidity, effervescent products are usually packaged in high barrier
foil and/or polymer films or in heavy-wall jars that contain desiccant packs.Since it is the source of the carbon dioxide, the carbonate salt is a
key material in an effervescent formula. The most commonly used carbonate salts
are sodium carbonate (soda ash) and sodium bicarbonate (baking soda).Sodium carbonate has a lower percentage of CO2 than bicarbonate and
since it requires 2 moles of acid per mole of the salt, it is slightly more
difficult to neutralize than baking soda. However, these attributes cause
products that are formulated with carbonate to be a bit more stable than those
that contain only bicarbonate.Bicarbonate has a higher proportion of
CO2 than soda ash and, due to its ability to easily break down, releasing water,
products formulated with it tend to react more quickly and be less stable than
carbonatebased products.Both carbonate and bicarbonate have advantages
and disadvantages. Therefore, most formulations use a blend of the two. 50/50 is
a typical ratio that generally achieves good reaction time and acceptable
stability.Although the sodium salts are the most commonly used
carbonates, potassium and magnesium carbonate can be used successfully in
effervescent products. The other key component in an effervescent composition is
the acid. It reacts with the carbonate salt, releasing the CO2 gas.The
most commonly used acid is citric acid. It is low cost, easily available, very
soluble and since it is trivalent, has good neutralizing power.
… more -
Fumaric acid is also frequently used. Even though it is only divalent, fumaric acid is actually a more efficient neutralizer than citric acid on a weight basis. This can be seen by comparing the equivalent weights of these materials. However, fumaric acid is much less soluble than citric acid and thus gives a slower reaction than citric. Probably because of this difference in solubility, fumaric acid products tend to be a bit more stable than citric acid products. Adipic and malic acids are also commonly used in effervescent bath products. As with the choice of carbonate salt, the desired product performance and manufacturing method will guide the choice of acid. Not only will the choice of acid affect performance, but the ratio of acid to carbonate will also affect the product. In general, higher ratios of acid to carbonate will yield faster reactions. Also, higher ratios of acid will assure that the carbonate is completely reacted. If the acid does not at least stoichiometrically balance the carbonate, some carbonate will be left unreacted and it will settle to the bottom of the bathtub. In general, 1:1 weight ratios of acid to total carbonate are common. However, highly reactive, highly soluble systems can use acid to carbonate ratios as low as 1:10.
Beyond the reactive ingredients, aesthetic and functional materials are usually incorporated in effervescent products.
Fragrances and essential oils are virtually always included in these products. Typical levels are 0.5% to 3%. Product design and intended consumer use will guide perfume selection and level. Most fragrance houses can provide technical assistance in designing perfumes for use in effervescent products. They can formulate the oil to be compatible with effervescent bases by avoiding materials such as glycol solvents that may cause instability to occur by allowing partial dissociation of the acid or carbonate.
Color selection will depend upon the desired product performance and aesthetics. Most cosmetic colors work well in effervescent bases. If the product needs to color the bath water, use dyes. If coloring only the product itself is important, use lakes and/or pigments. Color stability, particularly in light, must be checked carefully.
Functional materials are included in virtually every effervescent product. The key is to choose materials that are anhydrous, otherwise stability problems will occur. Botanical materials such as freeze-dried aloe, chamomile extract in oil, and even dried flower buds and bulk herbs have been used in effervescent products. Levels used are generally less than 1 or 2%.
Emollient materials such as squalane, vitamin E, almond oil and many cosmetic esters are frequently incorporated, again, generally at 0.1% to 2%. Surfactants are used both as fragrance emulsifiers and as foamers. When used as emulsifiers, surfactants prevent the perfume oil from floating on the water’s surface. Many consumers prefer this since floating oil systems tend to be messy and hard to clean up. Typical emulsifiers are PEG-30 castor oil, Polysorbate 80 or 85 and Laureth 4. The precise choice will depend on the HLB of the perfume oil.
If surfactants are going to be used to create foam, special formulations are required to achieve consumer acceptable performance. Polymers can also be added to help modify skin feel and the feel of the bath water. Commonly used materials include Polyquaternium 10 and PEG. Levels are typically 0.2% to 3 or 4%. Binders are almost always needed to make good, solid effervescent tablets. Sorbitol, lactose and maltodextrin are usually used at levels ranging from 10-20%.
Process aides are materials that are added to help the powders flow more efficiently and to prevent sticking on the production equipment. The most frequently used of these materials are fumed silica, calcium silicate, cornstarch, and sometimes talc. In general, these materials work well when incorporated at only a few tenths of a percent. ”
Lab Rat”
In looking at your formula the thing I noticed that seemed like it may be a problem is the high percentage of salt! I would probably reduce that to at least half of that you are currently using and play with your formula until you get it right! I think it is getting in the way of the citric acid and sodium bicarbonate bonding together. JMHO
David
-
I’m back with some result from the experiment. So I tried coating each ingredients separately with oil as suggested, though it still had reaction.
Then I tried using sodium carbonate instead of sodium bicarbonate for one batch and for another batch I baked sodium carbonate for another half an hour, both batches had the reaction the same even though it seemed to be slower than when I used sodium bicarbonate.I wonder if the issue is as David mentioned that the evironment has to be perfect, the facility’s low humidity, better packaging etc, if that’s the case, I will try my best but I don’t have the mean to do it in a better facility than my house. Thank you for the helpful details about how to different ingredients.
Otherwise, if the case is that regardless of what I do, these 2 ingredients still react. My only and best option is try Gunther’s suggestion by using a divider which seems very complicated ? because if my product is all in one jar, I don’t know how I would do it, do you have any suggestions what kind of divider I could use? Or do I have to pack the acid separately?
I appreciate all of your guys’ guidance.
Sofie
-
@Sophie9 I’m on the Texas Gulf Coast and yes the humidity is a nightmare for bath bombs! I have a dehumidifier that really helps. If you run one in the room you are preparing your bombs in for a couple hours before making them that may help. I can remove almost a whole bucket of water after running one for a couple hours so that shows just how humid it is! You would probably have to heat the ingredients as before to remove the extra water and then package them airtight immediately. Just a thought. And if it doesn’t work for bath bombs you will probably still love your dehumidifier! I know I do?. Or only make them in December and January when the humidity is at a somewhat decent level! Texas humidity- so awesome for your skin but so bad for bath bombs☹️
-
@Colorfuljulie I should really try a dehumidifier, even not for the bath bombs, I will still use it to collect water for consumption ? It’s crazy how humid it is, a bucket of water after a few hours…
thank you for all the tips.
Log in to reply.

