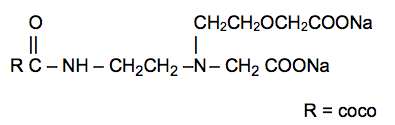Home › Cosmetic Science Talk › Formulating › Disodium Cocoamphodiacetate
-
Disodium Cocoamphodiacetate
Posted by David08848 on August 9, 2015 at 5:54 pmFolks,
Quick question! Are Disodium Cocoamphodiacetate and Sodium Cocoamphoacetate the same thing? I have been searching online and can’t find an answer.
Thanks!
DavidDavid08848 replied 8 years, 8 months ago 9 Members · 24 Replies -
24 Replies
-
Sorry, I don’t have access either. So many times I see different chemical names for the same product whether it is an INCI name or other type of chemical name so I don’t always know what the truth is and I don’t want to get any of this wrong.
-
Guys, I am rarely in favor of deception, but in this case I think an exception is warranted. Prospector wants to restrict access to legitimate businesses - but I think that so much of the information there is very valuable to beginning formulators - so, you should make yourself into a consulting business. Make a cheap website, get an email address that sounds professional, and re-apply for access.
-
@Bobzchemist: I’m working on it
 In the meantime, assuming you have access and you don’t mind, can you fill us in on the differences in the aforementioned chemicals?
In the meantime, assuming you have access and you don’t mind, can you fill us in on the differences in the aforementioned chemicals? -
OK I took a look and according to the brands the two are different. For instance Mackam™ 1C is the single form and Mackam® 2C is the disodium diampho form. Having said that I have used the mono form in formulae calling for the other, successfully.
-
Bobzchemist,
By “access” we mean that we get a message not allowing us to view those two items because we are not in Europe! I am a member of UL Prospector and have been for years and have ordered many samples and made connections with businesses there with whom I have done business.
Belassi, thanks! Sodium Cocoamphoacetate seems to be more readily available here in the US. and is available from resellers online when makes it convenient but expensive. What I would like to know are it’s characteristics in formulation. Chemical companies descriptions tend to tell the positive aspects not the less positive! Thanks for any insight you can provide!
David
-
I have used both interchangeably in formulations as a substitute for cocamidopropyl betaine. Functionally, I can’t tell the difference, which is why I’m curious if anyone has any other insights. Why would I use one over the other, besides cost, or is that the only difference?
-
Sodium cocoamphoacetate as far as I am concerned is a high quality surfactant that has an emulsification ability far higher than CAPB. For instance when adding the fragrance oils to a shampoo I always mix them with the SCA component first because I know I’ll get perfect emulsification. SCA is synergistic in ionic shampoos and can help form very thick mixtures. I like its foam - way superior to CAPB. Of course it is a lot more expensive.
-
Belassi and thebrain,
More pieces to the puzzle! Good to know that it performs well and has superior foam! I can see the cost being a problem but perhaps that is why I see formulations with both Sodium Cocoamphoacetate and CAPB in them? If it helps create a product that is more costly to make then just demands a higher price and appropriate presentation!Thanks again for the input! Much appreciated!
David -
Folks,
I am finding both Sodium Cocoamphoacetate and Disodium Cocoamphodiacetate as secondary surfactants but I am also seeing both listed as primary at the rate of 1-30, 4-40, up to 50% and others! Usually chemical companies are somewhere in the ballpark but in this instance they are all over the place? How common is this?
Thanks!
David -
@Bobzchemist and anyone else who’s interested. I set up a deal with Prospector that if use Chemists Corner as your company name when you register they will approve you.
-
that didn’t work but search on both compounds with google image and you will see there is a difference
-
Thanks David! I did find some things that indicated that these are two different substances. I also noticed that there were quite a few product ingredient labels on Google Image! Another helpful resource, thank you!
David
-
Oh thats great news @Perry, what shall we write for official email which they ask for??? Appreciate all your efforts Perry.
-
@Perry i tried using chemist77@chemistscorner.com
 not working
not working
I am trying to use my personal mail id but it says use company id nd which if i use makes life difficult as i work for a chemical trading co nd the moment they see it they restrict me to 75% site usage which defeats the whole purpose of being on ulprospector. -
Folks,
I got a sample of Disodium Cocoamphodiacetate yesterday and boy it is thick! I am still having some problems finding out info on Sodium Cocoamphoacetate and Disodium Cocoamphodiacetate! Even the info that came along with the sample did not list the active percentage although it did list solids at around 47-49%. Several listed “N/A” for the active percentage!! I’ve also tried finding out the difference between the two but haven’t had any success, so far!
David
-
having used both, I would say there’s no significant difference between them
the only real reasons I’d hesitate to use either of them would be the fatty/soapy smell they impart to the product, and their tendency to go yellow over time -
Good to know! I have an “older” sample that has become gold but I don’t have a problem with it. As far as the fatty/soapy smell, I’ll have to open it and check it out but since I am going after a “soapy” type of body wash, it may not be a problem. It also will be a secondary surfactant in the formula so I’ll give it a test run with one of the formulas and see how it works for me.
Thanks!
Log in to reply.