
Doreen
Forum Replies Created
-
Belassi said:Or perhaps use a hot air blower to simply remelt the top layer.
This is what I always do with lipbalms that have a bit of an indentation (I use a hot air gun). It also gives a nice shine.
-
@MJL
I think what Pharma means is that glycerine isn’t soluble in oil, isn’t lipophilic or doesn’t have lipophilic parts, so it won’t have as much effect as for exampe a surfactant in a ‘soap’ will have. That it is only just slightly better than water. -
em88 said:(…) Why do people bother to help when they don’t like doing it,resulting in the end being impolite?
My thoughts exactly!
-
@em88
You’re a pharmacist too if I remember correctly. You can ask Perry to put it behind your name, like Pharma has.
I thought I’d mention it as I know that you have so much professional knowledge. -
@PetalPoppet2309
A magnetic stirrer can be very handy to dissolve substances, I love to use it, but it isn’t strong enough (by far) to mix emulsions! -
@MarkBroussard
The 0.2% graph is shared in the Making Skincare Facebook groups of Jane Barber and it is adviced there due to ‘pro-oxidative’ reasons not to exceed 0.2%.
Instead of simply accepting it as truth, I should’ve studied the graph better myself. Lesson learned. -
ngarayeva001 said:(…) It might work as pro-oxidant at this concentration. (…)
This probably isn’t true. I know I’ve been sharing that graph several times, but upon closer look, it doesn’t say much. In any case nót a substantial raise by far. I too should’ve studied it better.
-
You really need to measure by weight and % (w/w). No idea how big your cup is!
-
Pharma said:LoL! No, botox doesn’t corrode metal but brain cells :blush: .The way you wrote it I was under the assumption that you probably might not know why it corrodes or think that it’s hypochlorite as is. Doesn’t really matter… I prefer hydrogen peroxide or in situ generated performic or persulfuric acid if I really have to get something clean. Although, I have to admit, the air gets somewhat unbreathable when using 30% H2O2 or performic acid.You could try out an ozone generator. It doesn’t affect the steel work bench (it only annihilates everything else in the room, but not the steel work bench). A doctorate student made that experience… except metal things, EVERYTHING else crumbled to dust when touched! It was like in a Hollywood movie when sunlight hits a vampire. It was hilarious (for those who hadn’t their work ozonised).
Thanks for thinking along! An ozone generator… reminds me somehow of a neutron bomb, destroying living tissue but leaving buildings intact. Yes I know, stupid comparison, but I watched a documentary about it not long ago.
I know from higher up they’re busy changing protocols on i.a. the Na hypochlorite causing corrosion. And yes, due to the chlorine! Weird right? I mean if you get a whiff of sodium hypochlorite, you’d never guess chlorine! Totally free from odour! :yum:


Also a change on the 70% IPA we use to sanitize the whole benches (I indeed have heard rumours of using H202 one week then IPA the other).Another shitty thing regarding nasties. The lab recently has found a fungus in this particular clean room where toxic steriles are made (mostly chemos). Fungi are the biggest horror scenario here as you can imagine.
No idea what the plans are yet…@Fekher
Ok, thanks for mentioning! I think I’ll stick with my metallic spatulas as I love to use these!@Dtdang
You’re welcome! -
ngarayeva001 said:@Pharma (…) By the way thank you very much for your input in my threads as well as in others. It’s great to have a pharmacist who is willing to share their knowledge (…)
I second this!
-
@Pharma
I have no idea what the level of iron is in my toner, if it would be as low as 10 ppm I would be very glad.
I use 5 ltr jerrycans of water that, according to the label, should be distilled ánd deionized, but the levels of trace metals aren’t mentioned. It’s the cheapest choice for me (the prices of distilled water in DIY shops here are outrageous).And then the salicylic acid. I don’t know the precise equivalent of the chemical purity grade that’s used in the US, but I think the salicylic acid that I used to buy was comparable to ‘lab’ (“A chemical grade of relatively high quality with exact levels of impurities unknown; usually pure enough for educational applications. Not pure enough to be offered for food, drug, or medicinal use of any kind.” Source).
Since a week I use Curcylic 40 by Vantage Specialty Ingredients, which also contains cocamidopropyl dimethylamine.These are the two main reasons that I use a chelating agent in it.
Edit: I think the grade of the SA is ‘purified’, a level below ‘lab’, but I’m not sure.
-
@Pharma
You’re right, it is indeed a shame that no concentrations were mentioned and no control group with Bronopol alone was used.
Does Bronopol have (strong) antifungal activity then? I thought that it was mainly antibacterial?About the lecithin that I use in some of my emulsions: do you think I could use parabens without worrying about these being inactived by the lecithin?
I use 1-3% Rovisome CE Plus (contains 10-25% lecithin) and 0.1-0.5% Phytrox LTR15-IP MB (lecithin is mentioned first in the LOI).
I would then use the maximum of the recommended concentration of the paraben containing preservative, like 1% Germaben II or 1% Phenonip for example. -
-
The fishy smell is typical for cationics.
-
@Pharma
Thanks for your extensive answer! Extremely interesting topic.
No I don’t use the GDL to disperse pigments in colour cosmetics. :joy:
It’s for a simple salicylic acid 2% exfoliating toner that I have been making for years with disodium EDTA and without much else in it.
I’m not even adding the EDTA from a microbial point of view, but mainly to keep the salicylic acid free from complexes. (Suffice it to say that the toner is well preserved of course.)Re: phytic acid. I’ve read an article about several bacterial species that are able to produce the enzyme phytase.
I’ve read an answer of a manufacturer of phytic acid containing products somewhere on this forum which was hardly satisfactory, so I’m sceptic. -
@Pharma
Thanks so much for your answers!
Glad to read I can keep on using them.
@Dtdang
The spatulas are made of stainless steel, but even stainless steel can oxidize over time, especially when (strong) oxidizers are used.
One of the stainless steel laminar airflow (downflow) benches at my work needs to be neutralized with a sodium hypochlorite 5% solution every time when Botox injections are prepared in it (even when there hasn’t been clearly visible spillage). The bench looks awful now, all rusty!
After a while it is replaced by a new one. The costs must be enormous…
To think about all the precautions we take and there are even ‘Botox parties’ by (mostly) women at home! They obviously have no idea what they’re dealing with! :#Edit: typo
-
I don’t know if the word that I used is the right translation. :#
But what I mean is this. Standard pharmacy utensils:

I am used to preparing emulsions, ointments etc. with a metallic spatula and ‘mortar scrape cards’:
I tried to work with plastic spatulas, but I can’t work as precise with those as I can with metallic spatulas (plastic ones are too thick, too much loss of product and thus active ingredient).I sincerely hope that I can keep on using the metallic kind with vitamin C derivatives! Thanks in advance for answers!
-
Pharma said:I wonder why you have an issue with that anyway???
I don’t have issues with it. I just wanted to know, to prevent useless SA-iron complexes. If iron complexes are present, would that be visibly noticable? Can I detect it without performing special tests?
I haven’t worked with GDL before, so I know nothing about it.
I can get disodium EDTA for example, but it’s much more expensive for me to get it here (shipping costs mainly). The only other options I have regarding chelating agents is phytic acid and its sodium salt.
-
Could you press Capslock please?

-
@chemicalmatt
You have never noticed parabens becoming less effective due to the lecithin?Re: Bronopol. What I found so interesting about the graph I shared is that it seems to ‘protect’ the parabens from becoming inactive due to the nonionics.
Are there restrictions that it can’t be used anymore?
I’ve never used it so I don’t know anything about it. -
@Belassi
I don’t know if it will relieve the symptoms of atopic dermatitis specifically, but this product has been recommended today by our vet (for dry, itchy skin). It’s a blend of ceramides, fatty acids and cholesterol, according to the manufacturer.
(Although I don’t see any fatty acids mentioned in ‘Composition’ in this leaflet?!)
I thought I’d mention it, maybe it can give you some ideas.
@Perry has written a very interesting article about ceramides by the way.Did you find a supplier in Mexico for vitamin B12?
I haven’t had time yet to make the B12 cream myself, but as soon as I have results, I’ll happily share them with you. -
@PetalPoppet2309
A good airless dispenser is also protected from air and contamination around the opening/’outlet’ of the pump: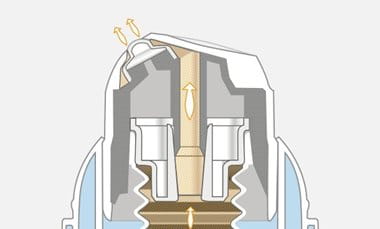

If you’re a homecrafter, Aliacura in Germany sells these at very reasonable prices. They ship worldwide. The company is run by two pharmacists and their price - quality ratio is simply outstanding.
-
@MarkBroussard
Thank you very much for your answer!If I use the Rovisome CE Plus or the Phytrox LTR15-IP MB in a formula, I use other preservatives than (combination products with) parabens, I just don’t want to take the chance that they might become ineffective because of the lecithin in it (and the only tests that I can perform right now on my formulas are plate counts).
Regarding interactions with parabens. I’ve read in a research about the paraben - non-ionics interaction that the addition of bronopol seems to keep the parabens working for (at least) some non ionics. Very interesting.
Table F.2.9. Preservative compatibility with nonionic surfactants
P = Parabens alone.
P+PE = Combinatinon product with parabens and phenoxyethanol.
P+PE+B = Combination product with parabens, phenoxyethanol and bronopol.Non ionic | Complete Inactivation | Significant Inactivation | No Inactivation
PEG-5
stearyl ether P, P+PE, P+PE+BPEG-15
stearyl ether P P+PE P+PE+BCeteareth-20 P, P+PE P+PE+B
Polysorbate 60 P P+PE P+PE+B
( https://www.sciencedirect.com/topics/immunology-and-microbiology/propylparaben , topic: Biocides)@Kirkland after this I won’t hijack your thread any longer.

-
Doreen
MemberJune 21, 2019 at 9:21 am in reply to: Formulating Salicylic Acid in a Facial Cleansing TonerBelassi said:Yes. If the formula I suggested isn’t organic enough, first you will need to hire a chain saw and find some willows. Near water, they are, usually.

@ngarayeva001
Why room temperature? I always heat the salicylic acid mixture to 70C and also add it to the water phase at 70C. After that I raise the pH to at least 3-3.2 when it’s still hot. If I wait too long with adjusting the pH in the cool down, the SA starts to precipitate. Later at room temp I do the final adjustment.

